Today most of the laptops, mobile phones, digital cameras are powered by the energy efficient Lithium -ion batteries. These batteries are approaching to their energy capacity limits. The conventional Lithium Ion batteries have poor energy density because of the dense and heavy Lithium Cobalt Oxide as positive plate.
Rechargeable lithium batteries are currently comprised of a graphite negative electrode, an organic electrolyte and lithium cobalt oxide as the positive electrode. Lithium is removed from the layered intercalation compound (lithium cobalt oxide) on charging and re-inserted on discharge.
A revolutionary battery has been developed by Peter Bruce of the University of Strathclyde and Newcastle, which increases the efficiency of Lithium batteries to 10 times. The main principle of this battery is that it takes oxygen from the air during discharge, replacing the chemical constituent used in rechargeable conventional Lithium Battery. The electrode is made of Porous Carbon, which is cheaper and light weight than Lithium Cobalt Oxide.
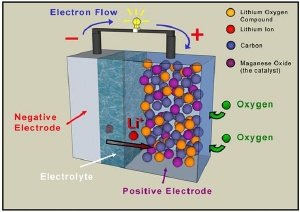
Key advantage:
High capacity to weight ratio roughly eight to ten times that of a conventional Lithium Ion battery.
RELATED POSTS
View all
